By Lambert Strether of Corrente.
In our continuing focus on CDC’s HICPAC (Healthcare Infection Control Practices Advisory Committee), we’ve looked at their upcoming August 22 teleconference, where it seems likely that the conflicted commitee will downgrade HCW protection from airborne diseases from N95s to “Baggy Blues.” In this post, I want to look at the regulatory auspices under which HICPAC meetings are held. Perhaps some clever lawyer will be able to work these ideas up into a brief that will prevent whatever HICPAC agrees on August from becoming CDC guidance.
HICPAC meetings are held, says CDC, under the aegis of the Federal Advisory Committee Act (FACA), described by the Congressional Research Service (CRO):
Federal advisory committees are created by Congress, Presidents, and executive branch agencies to gain expertise and policy advice from individuals outside the federal government. Many federal advisory committees are subject to the Federal Advisory Committee Act (FACA; 5 U.S.C. Chapter 10), which includes statutory meeting and transparency requirements. The Committee Management Secretariat (hereinafter “”Secretariat””) of the General Services Administration (GSA) is responsible for matters relating to advisory committees subject to FACA. In the Final Rule, GSA stated [w]hile FACA is not a public participation statute, it directly affects how the executive branch is held accountable for the use and management of Federal advisory committees as a major means of obtaining public involvement….. In order for Congress and the public to be kept informed on the activities of advisory committees, FACA provides that meetings be open and available to public inspection. GSA’s discussion of meeting accessibility includes the requirement that any member of the public be permitted to file a written statement with the advisory committee, and be permitted to speak or address the advisory committee in accordance with the agency’s guidelines.
This is laudable guidance, with which HICPAC is currently out of compliance in three areas: the Minutes, the Agenda, and the “Membership Balance Plan.” The first and third of these violate the letter of FACA; the second violates the spirit. Let us take each in turn.
Under FACA, HICPAC Meeting Minutes Must Be Posted, But Are Not
According to the GSA (“The Federal Advisory Committee Act (FACA) Brochure“) requires that “federal agencies” (like CDC) “sponsoring advisory committees” (like HICPAC), must “Make available for public inspection, subject to the Freedom of Information Act, papers and records, including detailed minutes of each meeting….” Here is the HICPAC site:
Where are the 2023 minutes? (To be fair, the CRO writes that the GSA’s FACA “guidance does not indicate that meeting minutes must be published in advance of the committee’s next meeting date.” To be fair, the CDC says although HICPAC meetings may occur “up to” 8 times a year, this year (at least according to the Federal Register) the only previous 2023 meeting — oddly; was there nothing to discuss (in public?) — was on June 8. Surely 67 days is enough time for a large Federal agency to prepare meeting minutes?)
HICPAC Has Obfuscated the Meeting Agenda, to the Detriment of the Public
According to the CRO, the FACA Final Rule requires advisory committee meeting notices to be published in the Federal Register at least 15 calendar days in advance of the meeting. The requirements;
- the name of the committee;
- the time, date, and place of the meeting;
- a summary of the agenda;
- a statement of whether the meeting is open to the public or will be closed pursuant to the Government in the Sunshine Act (Sunshine Act; 5 U.S.C. §552b); and
- the name of the Designated Federal Officer or other responsible agency official who may be contacted for additional information concerning the meeting.
There is in fact such a Federal Register announcement for HICPAC’s August 22 meeting, and it includes the agenda:
The agenda will include the following updates: The Healthcare Personnel Guideline Workgroup; Isolation Precautions Guideline Workgroup; National Healthcare Safety Network Workgroup; and Dental Unit Waterlines Guideline Update. Agenda items are subject to change as priorities dictate.
(Note the Schrödinger’s Agenda wording at the end.)
But now let’s take a look at the HICPAC “Meeting Information” page at CDC. No agenda:
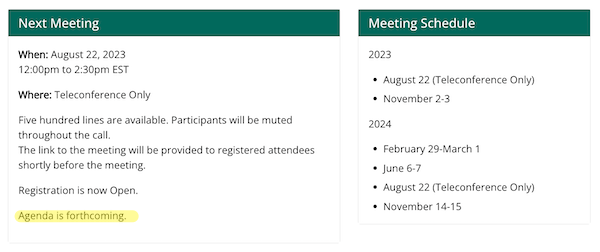
CDC’s own site contradict’s its Federal Register announcement. Although presumably the Federal Register is controlling, how is this “open” or “transparent”?
Further, the “Meeting Information” page includes the following Kafka-eque registration section:
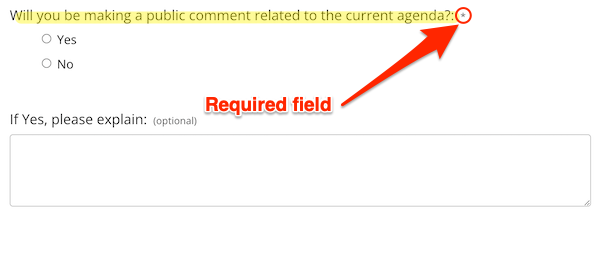
It’s a very nice touch to ask a member of the public if they “will be making a comment related to the current agenda” when there is no agenda to be had! Even better — moving into “dark pattern” territory — answering the question is required (via the “*”). What is the user to do? Type in “Yes, but I don’t know what”? “No, until you tell me what the agenda is”? Abandon the form in frustration?
Under FACA, HICPAC’s “Membership Balance Plan” Must Be “Fairly Balanced” in “Points of View” But Is Not
Here is the GSA guidance on what a “Membership Balance Plan” must look like:
Section 5(b)(2) of the FACA requires “”…the membership of the advisory committee to be fairly balanced in terms of the points of view represented and the functions to be performed by the advisory committee.”” The corresponding FACA regulations reiterate this requirement at 41 CFR § 102-3.30(c), and, for discretionary committees being established, renewed, or reestablished, require agencies to provide a description of their plan to attain fairly balanced membership during the charter consultation process with GSA (41 CFR § 102- 3.60(b)(3)). The document created through this process is the Membership Balance Plan
CDC’s HICPAC does, in fact, have a “Membership Balance Plan.” Unfortunately, it’s not accessible to the general public. GSA elaborates on points of view:
The FACA regulations offer guidance in achieving a balanced Federal advisory committee membership, which include considering: (iii)The types of specific perspectives required, such as those of consumers, technical experts, the public at-large, academia, business, or other sectors; (iv) The need to obtain divergent points of view on the issues before the Federal advisory committee…
It’s plain as day that HICPAC’s membership is not “divergent” as FACA understands the term; every one of its members is affiliated either with a hospital or a medical care facility. Conflicts aside, the controlling assumption can only be that hospital staff have nothing to learn about infection control from anyone outside their milieu. Surely such mental and ideological inbreeding is exactly what an open and transparent process seeks to prevent? (“Sunlight is the best disinfectant,” as they say.) CDC has an entire institute, NIOSH, with expertise in “respirators and masks.” Are we seriously to believe that NIOSH has nothing to contribute to HICPAC on masking? Or ventilation? Or training? Even if NIOSH doesn’t sit on the committee, why are they not invited experts? Why on earth does HICPAC’s draft “Isolation Precautions Guideline Workgroup” deliverable (PDF), which is driving masking policy and the infection model for HICPAC, not even mention NIOSH?
Infection Control Today comments:
HICPAC’s composition was a concern for many commentators. As stressed by M.K. Fletcher, MSPH, BS, the committee should include “”aerosol scientists and ventilation experts, respirator protection experts, and industrial hygienists.””
From the People’s CDC:
More than 900 occupational safety, aerosols scientists, public health, and medical experts have already written to new CDC director Mandy Cohen telling her that CDC/HICPAC must correct their review to reflect the science of aerosols transmission and their decision-making process to include patient advocates, aerosols scientists, union representatives and occupational safety and health experts.
Redress from HICPAC’s Designated Federal Officer?
FACA, says the GSA, requires a “Designated Federal Officer“:
In addition, a Designated Federal Officer must be assigned to each committee to:
- Ensure compliance with FACA, and any other applicable laws and regulations;
- Call, attend, and adjourn committee meetings;
- Approve agendas;
- Maintain required records on costs and membership;
- Ensure efficient operations;
- Maintain records for availability to the public; and
- Provide copies of committee reports to the Committee Management Officer for forwarding to the Library of Congress.
The CDC says that HICPAC’s Designated Federal Officer is Michael Bell, M.D. From the Federal Register, here is Bell’s contact information, as of 2020:
FOR FURTHER INFORMATION CONTACT: Michael Bell, M.D., Designated Federal Officer, HICPAC, Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC, l600 Clifton Road, NE, MS H16-3, Atlanta, Georgia 30329-4027; Telephone: 404-639-4000; Email: hicpac@cdc.gov.
GSA’s FACA guidance, above, permits Bell, in his capacity as Designated Federal Officer, to call meetings, and therefore not to call them (or to immediately adjourn them, if called). I suggest that Bell exercise his powers to “ensure compliance with FACA”, and postpone the next HICPAC meeting until (a) the previous meeting’s agenda is up, (b) the meeting’s agenda is posted on the CDC’s website (with, ideally, the Schrödinger’s clause removed, and (c) HICPAC’s committee composition has “divergent” points of view.
APPENDIX
According to Infection Control Today: “Important [HICPAC] votes are held before and not after public comment.” If true, that’s both a bad look, and bad. The Designated Federal Officer should stop this as well.


