Investigative reporter Alex Berenson is a controversial source on matters Covid. He generally hews to Great Barrington Declaration views, that Covid is not a serious pathogen, that the lockdown were unnecessary and a violation of individual rights, that masking does not work. Nevertheless, he’s also been diligently investigating studies that question the safety and efficacy of the Covid vaccines, particularly the mRNA vaccines. And he appears to have a serious finding, based on Moderna’s own claims versus the data in a paper that the journal Vaccines published in February 2021.
The very short version is that Moderna maintained, both in its clinical trial data to the FDA, and in the Vaccines paper that there were no “serious adverse effects” in a trial group of 400 versus a control of 200.1 In fact, there were 14, including three miscarriages, seven during the placebo-controlled phase of that trial (the first shot), seven more with a booster (no typo, Moderna started trials of a booster in January 2021). As Dima at Military Summary is wont to say, “That’s a lot.”
Mind you, Moderna and the FDA misrepresented vaccine safety by hiding these injuries. The Journal Vaccines published a paper in February 2021 on the Moderna Phase 2 study, claiming there were no severe adverse effects; the data showing otherwise was made public only at the end December 2022.
This information is potentially significant since some lawyers claim that understating adverse effects would amount to fraud and would void the liability waiver the Federal government issued under the Emergency Use Authorization. If Berenson’s finding is deemed to be significant, we may seem some vaccine injury cases filed to try to surmount this indemnification.
The Moderna study in question was its Phase 2 trial. Phase 1 trials are very small and are to investigate safety and dose levels. Phase 2 trials usually involve more participants and gather more data about safety as well as collect information about efficacy. If Phase 2 goes well, the Phase 3 trial administers the treatment to a much larger population. Only after the Phase 3 trial can the drug maker apply for FDA approval.
In Berenson’s article, he contrasts Moderna’s statements in the Vaccines paper (emphasis his)….:
with the data made public only recently (the table excerpt is a bit confusing; the top row presents total severe adverse events as seven; the rows below break out only cardiac disorders):
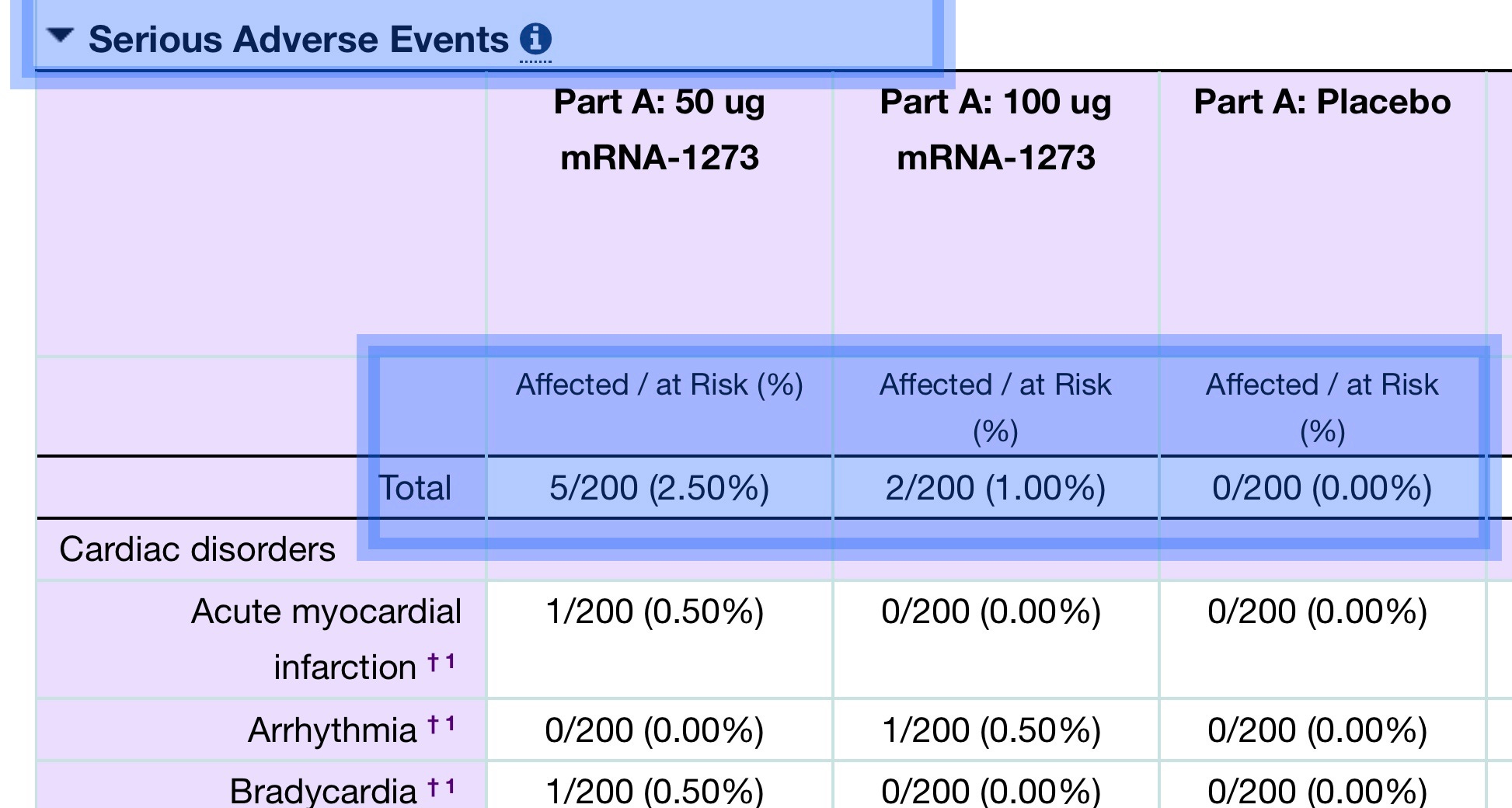
Berenson also notes:

I wish Berenson had presented a timeline with populations, number of jabs, and side effects. As he explains, the data on this population of 600 of the initial subjects + controls, which became 550 who took the shots because the control population was soon offered the opportunity to get the shot.2
Here is the guts of his post:
Because it started in May 2020, two months before the big pivotal trials, the P201 trial had a placebo-controlled arm for longer than any other mRNA Covid jab trial.
Regulators and public health experts had always believed that if the shots had side effects, they would be evident quickly. Moncef Slaoui, the head of Operation Warp Speed, the government program that helped develop the jabs, told federal officials that 99 percent of side effects from vaccines became visible within 60 days.
But the gap in serious side effects between placebo and vaccine recipients widened for as long as the P201 trial progressed, belying Slaoui’s assumption.
The P201 results raise the question of what might have happened if regulators had forced Moderna and Pfizer to continue collecting placebo-controlled safety their much larger pivotal Phase 3 clinical trials for even two or three more months in early 2021…
…we do not have and will never have long-term safety data comparing people who received the jabs with those who received a placebo. The P201 safety dataset is the longest publicly available.
—
It is not comforting.
The miscarriage reports are striking, given the small number of women of childbearing age – almost certainly no more than 150, and possibly closer to 100 – in the trial.
Worse, in its final report on side effects, Moderna disclosed a third miscarriage in 2021 in a P201 trial subject after she received a booster shot. (Moderna began testing boosters on the P201 trialists in January 2021, at a time when the public was being told that Covid boosters would probably not be needed for years, if ever.)
In all, in its final report, Moderna counted a total of 11 people who suffered serious side effects among about 350 people who received the jabs plus a single booster shot.
In addition, two placebo recipients suffered serious side effects after the trial was unblinded and they took the jab. Finally, in the trial’s final phase, Moderna gave 60 people a second booster; one of those 60 developed stage four cancer.
In all, 14 people who received jabs in the trial suffered serious side effects.
These trialists were a generally healthy group – healthier than the Phase 3 trialists, or average Americans. For example, Moderna excluded people with diabetes from P201, and few people in the trial were obese.
Recall that NC regular IM Doc, among his many claims to fame, was on an Institutional Review Board for over a decade and its chair for about five years. From the FDA:
Under FDA regulations, an Institutional Review Board is group that has been formally designated to review and monitor biomedical research involving human subjects. In accordance with FDA regulations, an IRB has the authority to approve, require modifications in (to secure approval), or disapprove research. This group review serves an important role in the protection of the rights and welfare of human research subjects.
That means IM Doc is a very seasoned interpreter of clinical trials and their use of statistics. He pointed out that Emergency Use Authorization process bypassed the Institutional Review Board process, eliminating a critically important patient protection.
Readers may remember that IM Doc was very critical of the initial data presentation of the Pfizer vaccine and the unheard of cheerleading in the related New England Journal of Medicine editorial. As IM Doc wrote then:
Unfortunately, this study from Pfizer in the latest NEJM, and indeed this whole vaccine rollout, are case studies in the pathology [Dr. Marcia] Agnell described [in her seminal 2009 article, Drug Companies & Doctors: A Story of Corruption]. There are more red flags in this paper and related events than present on any May Day in downtown Beijing. Yet all anyone hears from our media, our medical elites, and our politicians are loud hosannas and complete unquestioning acceptance of this new technique. And lately, ridicule and spite for anyone who dares to raise questions.
IM Doc elaborates on his concerns in this and two later posts.
He weighed in on the Berenson article:
Well – it is finally starting to come out. There is indeed an importance to those of us who have been around the block for decades. Who can recognize patterns that are very abnormal.
I have now had 4 midterm miscarriages and possibly one other – since the vaccines have been out. We were very early on, here, strictly a Moderna town. There were zero mid term miscarriages in my practice in the COVID year pre vaccine of 2020.
All of these women had one thing in common – sometime in the previous 3 months before the miscarriage, they had either the initial series or a booster.
4-5 mid-term miscarriages in the past 2 years.
The preceding 30 years of my career – only one – and that was not spontaneous – that was after a car wreck.
It is also interesting to note that I have not had any of these issues for the past 9 months or so – corresponding to the time when en masse, the population quit taking these boosters like they had been. While COVID itself is still raging on.
It is important to realize that IRBs would have caught on to these issues and dealt with them. There is a reason the EUA legislation removes them from the mix.
The more disturbing thing which must be ran down is if the accusations in this article are true – that both Moderna and the FDA have been falsifying data.
That could get nuclear. Especially if the trend holds up with all the barracuda injury lawyers smelling blood in the water.
I find it continually fascinating that the national press continues to ignore every bit of this. As the Ethics professors of the 1990s were so keen to point out – it really does make a difference when 50% of your revenue comes from Big Pharma – Big Pharma will be protected.
Let me stress as IM Doc does that mid-term miscarriages are (normally) exceedingly rare. They nearly always happen in the first trimester when the mother and fetus are working out how to live together. He described longer-form how devastating the loss has been to two generations of one of the affected families, including triggering substance abuse.
And IM Doc again is correct about the press. Berenson’s piece has not gotten much traction on Twitter and only some re-reporting in right-wing outlets. Perversely, the best thing that may have happened to him is Streisand effect by virtue of Instagram banning the piece:
Who’s got two thumbs and will be sending @instagram a demand letter, prior to filing a defamation lawsuit over their libelous smear on my Stack?
This guy! (Technically this guy’s lawyer, @jlawrencenc).
I am SICK of censorship. I know my story is correct and true. So will a jury pic.twitter.com/LflWM0d4hd
— Alex Berenson (@AlexBerenson) April 22, 2023
Berenson did solicit comment from Moderna and got no response. One could criticize Berenson’s article for being a bit hard to follow but the facts and timeline are complicated. But it appears to be sound and I hope his story does get attention. Sometimes it takes time….witness the Discord leaks.
____
1 The reason for a study group 2x as large at the placebo group was that Moderna was testing two different dose levels.
2 The numbers remain confusing. Berenson says 158 of the 200 in the placebo group accepted the later offer to get the vaccine. I assume the disparity of 8 (400 + 158 ≠ 550) is due to dropouts. Also note, as IM Doc said with some ire at the time, that deliberately eliminating the control group so soon after a clinical trial is unheard of.


