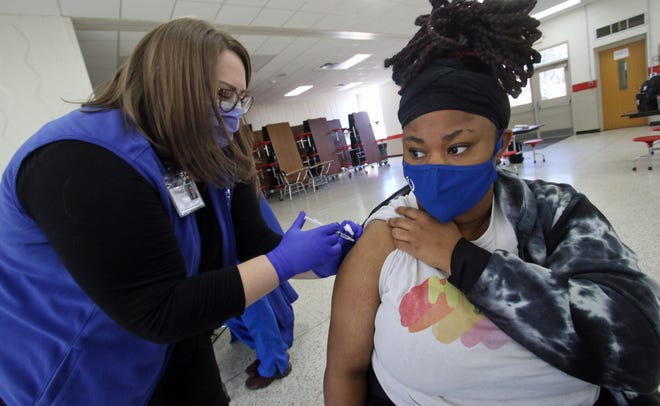
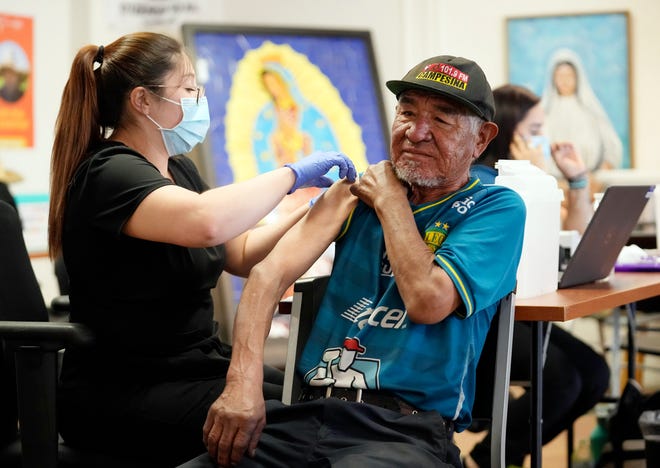
A federal advisory panel voted 19-2 Tuesday to reformulate COVID-19 booster shots for the fall to more directly target the omicron viral variant.
Members of the Food and Drug Administration’s advisory panel supported targeting omicron’s BA.4 and BA.5 subvariants, which now account for about half of the COVID-19 cases in the U.S. and which have been increasing in recent weeks as other subvariants fade.
Committee members also largely supported the idea of aiming at both the omicron strain and the original one in a single vaccine, which the companies said they can provide.
Panel members said they had far less data about the new boosters than they wanted. But they said they were comfortable the changes would be safe and felt compelled to try to prevent as much severe disease as possible by further targeting the vaccine.
Cases are expected to surge again toward the end of this year, as they did in both late 2020 and late 2021.
MORE:Most pharmacies in the US can’t give your infant or toddler a COVID shot. Here’s why.
Vaccine makers have tested new versions of their vaccines against the BA.1 version of omicron, but not against these more recent subvariants. But officials said it made sense to match the new boosters as closely as possible to circulating strains of the SARS-CoV-2 virus.
Only about half of Americans have received at least one booster dose and even those who got extra shots have less protection as time goes on, said Dr. Peter Marks, who heads the Food and Drug Administration division responsible for regulating vaccines.
“That combination of waning immunity, combined with the potential emergence of novel variants, during a time this winter when people move inside as a population increases our risk of a major COVID-19 outbreak,” Marks said in opening the day-long hearing. “For that reason, we have to give serious consideration to a booster campaign this fall to help protect us.”
Vaccine makers will need time to produce a new vaccine formula, Marks said, so a decision had to be made now for shots to be available by late October.
The current vaccines and their booster doses were developed to target the spike protein of the original SARS-CoV-2 virus, which began spreading around the world in late 2019 and early 2020. But the virus has evolved since then – through major variants called alpha, beta, delta and now versions of omicron.
VACCINE AND KIDS:Pfizer’s vaccine is safe and appears effective for kids under 5
MONKEYPOX:US to release at least 1.6 million vaccines by end of year
Pfizer-BioNTech, Moderna and Novavax have developed and tested in a small number of people new vaccine formulations aimed at the omicron subvariant BA.1, which was circulating last winter and which caused the biggest outbreak of the two-and-a-half-year pandemic. BA.1 has since been replaced by other omicron subvariants.
Not all the committee members agreed a change was necessary.
Two out of the 21 voting members voted against changing the shots, saying current vaccines are sufficiently protective. The companies lacked evidence, they said, that targeting omicron would make a real-world difference in preventing severe infections or saving lives.
Dr. Paul Offit, a vaccine expert at the Children’s Hospital of Philadelphia who voted against the change, said he thinks some people, particularly those who are elderly or immune compromised, will need boosters this fall.
But he was not convinced that omicron should be part of that vaccine.
“When you ask people to get a vaccine, the benefit should be clear because there’s always a risk,” he said after the meeting. “I just don’t think the benefits were clear.”
Data from the companies suggest that boosters of all varieties are “about equally good at reactivating our memory cells,” said John Moore, a vaccine and virology expert at Weill Cornell Medicine in New York City, who is not a committee member. “That’s why the variant boosters are not standouts as amazingly different from the (original vaccine booster), because they’re all doing the same thing.”
Moore said he was concerned the FDA officials were pushing the panelists toward a decision without adequate information.
“That’s not a good scenario when making a multi-billion dollar decision that potentially affects tens of millions of people,” he said via email during the meeting.
RESET EXPECATIONS:COVID vaccines are not meant to prevent all infections, experts say
In a statement released shortly after the vote, Pfizer said it specifically chose mRNA technology for its COVID-19 vaccine because it can readily be altered to address new viral variants.
“The data presented and discussed at today’s meeting underscore that we can swiftly put into place a program and validate the process,” according to the statement. “We believe that we’ve found possible solutions as we head into this next chapter of the pandemic and will continue to share our available data to health experts and regulators, in order to bring a omicron-adapted booster to the world as quickly as possible.”
The Vaccines and Related Biological Products Advisory Committee did not discuss the cost of changing the vaccine’s composition, but it will cost both the companies and the federal government.
Congress has so far declined to provide funding the administration has requested to pay for COVID-19 vaccines, treatments, testing and support. The money will run out later this year and the administration has been unable to negotiate with vaccine makers for supplies for the fall, potentially leaving the U.S. with too few vaccine doses to boost protections heading into the winter season.
“We need (additional) funding to make sure that every American who wants a bivalent vaccine, should one be authorized by the FDA, has the ability to do that,” Dr. Ashish Jha, the White House Coronavirus Response Coordinator, said in a Thursday news conference.
Contact Karen Weintraub at kweintraub@usatoday.com.
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.