Yves here. KLG unpacks a new paper that has gotten a lot of play in the popular press, which presents a new theory of why women get autoimmune diseases more often than men. He explains the research and explains why it’s a very credible piece of work. KLG also speculates why autoimmune diseases are on the rise and thinks ultra-processed diets are a likely suspect.
BTW my father had an autoimmune disease that is terminal, dermatomyositis.
By KLG, who has held research and academic positions in three US medical schools since 1995 and is currently Professor of Biochemistry and Associate Dean. He has performed and directed research on protein structure, function, and evolution; cell adhesion and motility; the mechanism of viral fusion proteins; and assembly of the vertebrate heart. He has served on national review panels of both public and private funding agencies, and his research and that of his students has been funded by the American Heart Association, American Cancer Society, and National Institutes of Health.
Today we consider the pressing medical mystery of why autoimmune disorders are much more common in females than males. Overall, 80% of patients with autoimmune disorders are female. These include Flannery O’Connor, Toni Braxton, and Selena Gomez, each of whom was diagnosed with lupus. Men who have suffered from lupus include the CBS News reporter Charles Kuralt and “My Favorite Martian” Ray Walston. This sex-biased distribution of autoimmune disease has long been a puzzle. Previous speculation has centered on hormonal differences between females and males as the underlying factor. Although perfectly reasonable, this has not yet led to a plausible mechanism for autoimmune disease.
A paper published in the journal Cell on 1 February 2024 has proposed a molecular mechanism for these sex-biased illnesses, including systemic lupus erythematosus (SLE; 9:1 females to males) and Sjögren’s disease; 19:1 females to males): Xist ribonucleotide proteins promote female sex-biased autoimmunity (NB: sex-biased, not sex-based; this paper is open-access, 34 pages in the pdf including some of the supplemental material). What does this imposing title mean?
From the Summary:
- Autoimmune diseases disproportionately affect females.
- The XX sex chromosome complement is strongly associated with susceptibility to autoimmunity. Females are XX, males are XY. XXY males (Klinefelter Syndrome) are more likely to have autoimmune disease than XY males.
- Xist (pronounced Ex-ist: short video, 0:00 – 4:15) long non-coding RNA (lncRNA; i.e., a long RNA molecule distinct from mRNAs that code for proteins, as in COVID-19 vaccines) is expressed only in females to randomly inactivate one of the two X chromosomes in each cell. This gene dosage compensation is essential, because only one sex chromosome should be active in any cell. X-inactivation is also called Lyonization after the geneticist Mary Lyon; Calico cat coat color is the result of random X-inactivation with each X chromosome carrying different pigmentation genes.
- The Xist ribonucleoprotein (RNP) complex comprising numerous autoantigenic components is an important driver of sex-biased autoimmunity (the archetypal RNP is the ribosome, the intracellular machine that produces protein using the mRNA template).
This graphical abstract outlines Xist-mediated autoimmunity (all scientific papers should include a graphical abstract):
How does this paper elucidate this mechanism? In plain English:
- An Xist lncRNA construct facilitated the assembly of RNP complexes that led to the production of autoantibodies associated with autoimmune disease when expressed in male mice.
- These male mice then developed lupus-like multi-organ pathology, while their immune cells resembled those of the normal female.
Moreover, in screens of patient samples:
- Human patients with autoimmune disease have autoantibodies to protein components of the Xist RNP.
- This demonstrates that the sex-specific lncRNA and its protein partners (Xist RNP) drive sex-biasedautoimmunity.
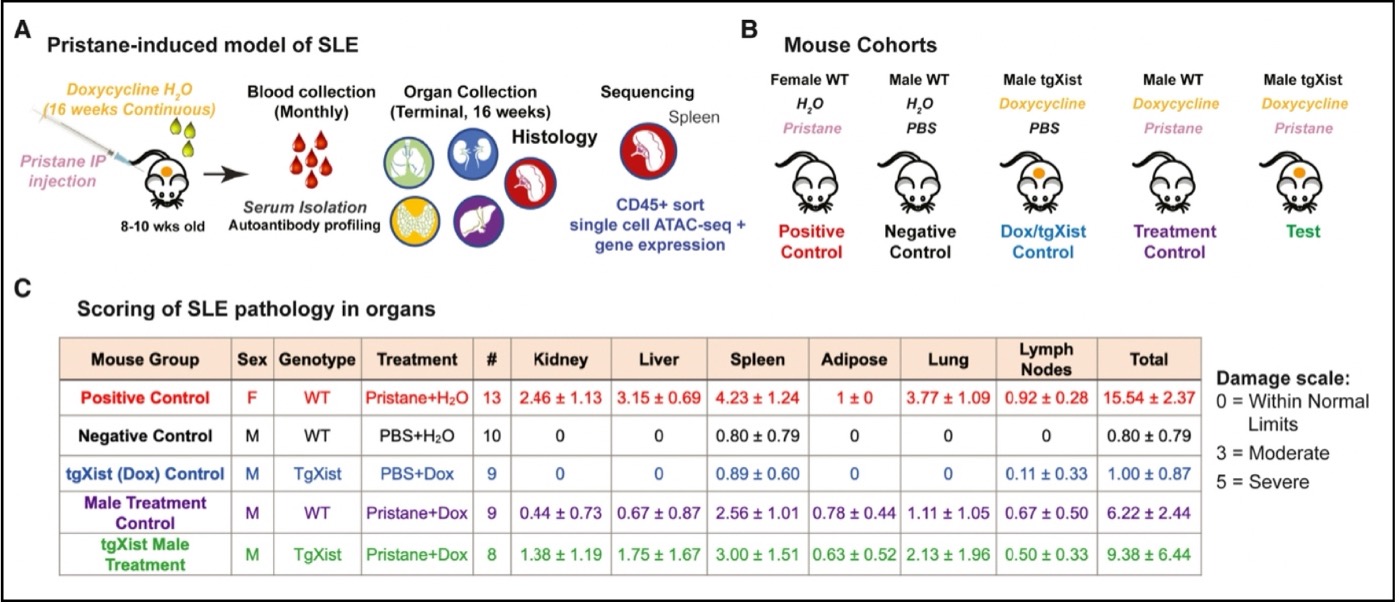
The experimental model (A) used here is based on a well-characterized mouse model of SLE and rheumatoid arthritis (RA) in which injection of pristane leads to SLE- and RA-like organ damage. Transgenic Xist (tgXist) mice in which the transgene is turned on by doxycycline (Dox) in their drinking water are injected with pristane to induce SLE [1]. Blood was then collected for autoantibody profiling. Organs (lungs, liver, kidney, spleen, adipose, lymph nodes) from experimental and control mice were also collected and examined for autoimmune damage, while immune cells were isolated from the spleen for further characterization.
The experiment (B): the positive control (1) is a wild-type female injected with pristane to induce SLE. The negative control (2) is a wild-type male injected with PBS (phosphate-buffered saline), which should not lead to SLE. The DOX tgXist control (3) is a male that expresses Xist but is not treated with pristane to induce SLE. The treatment control (4) is a wild-type male injected with pristane and doxycycline; pristane should induce SLE while doxycycline in the absence of the tgXist construct should have no measurable effect. The test (5) is a tgXist male in which the Xist transgene is induced with doxycycline plus injection with pristane to induce SLE.
The results (C) based on a score of SLE-associated organ damage: As expected, the female (1, positive control) treated with pristane develops severe SLE (15.54 total). The wild-type male (2, negative control) treated with PBS has no measurable SLE (0.80), and similarly the tgXist male (3, uninduced control, PBS + Dox, no pristane) has no measurable SLE (1.00). The wild-type male (4, treatment control, Dox + Pristane) develops severe SLE (6.22) but not as severe as the female positive control. The test tgXist male (5, tgXist, Dox + Pristane) develops SLE that is more severe than the treatment control male (9.38). Thus, females develop severe SLE when treated with pristane, which was expected. Males treated with pristane also develop severe SLE that is less damaging than that in females. SLE is more severe in males expressing the Xist lncRNA they would not normally have. The clear result is that the Xist RNP is associated with autoimmune damage. The test males (5) also have SLE-associated autoantibodies, which completes the current picture in the mouse model.
Still, the fair question is whether these data have clinical relevance for human patients with autoimmune disease. The answer to this is yes, as the excerpt below from Figure 6 shows that autoimmune disease patients experience increased serum reactivity toward antigens of the Xist RNP. Three autoimmune disease groups (DM, dermatomyositis; SSc, scleroderma; and SLE) were significantly reactive to 79 unique proteins in the array of autoimmune antigens, in comparison with a control consisting of samples from the general population.
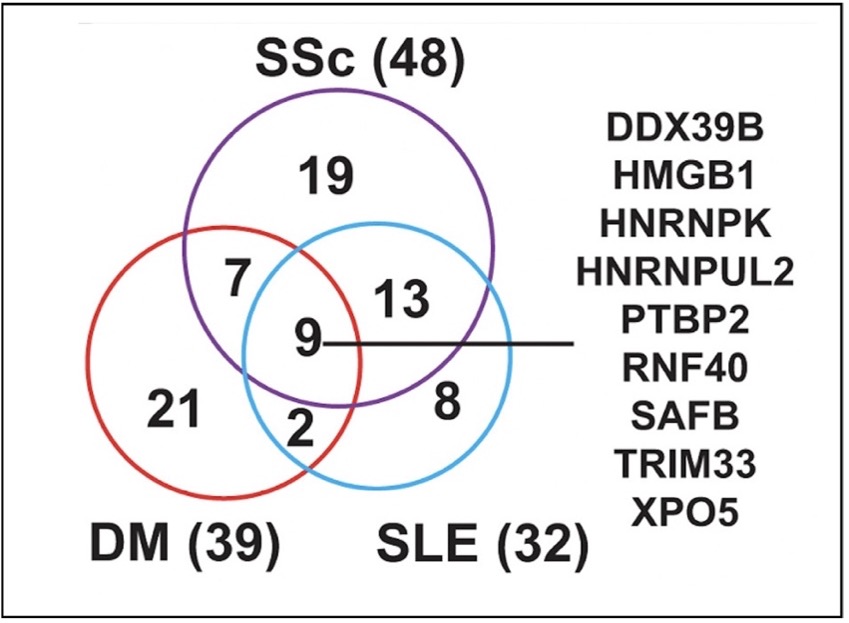
Twenty-seven (27) of these proteins were disease controls and 53 were associated with the Xist RNP. Of the 9 proteins common to all three diseases, 8 were Xist RNP proteins. In addition, 37 of the 53 Xist-associated proteins were part of the group of 118 high-confidence Xist RNP complex proteins previously identified. These results show that multiple proteins from the Xist RNPs are novel autoantigens in patients with DM, SSc, and SLE.
Finally, why is this research important? More than 100 autoimmune diseases have been described. They afflict 50 million Americans, or approximately 15% of the current population of 336 million and are a leading cause of death worldwide for women under the age of 65. These numbers are increasing. Thus, a deeper understanding of risk factors for autoimmune disease is critical if we are to develop interventions without practicing what Sir William Osler (1849-1919) called “a sort of pop-gun pharmacy, hitting the malady and again the patient…usually not knowing which.”
Autoimmune disease is heterogenous with overlapping traits, but as noted in this paper, “Our discovery of seropositivity toward multiple XIST-associating proteins in autoimmune patients introduces a novel antigen set with clinical potential for enhancing disease detection and monitoring, as autoantibodies are often detected prior to or early in disease onset.” While directed and effective interventions have not yet been identified, this cannot even be contemplated without a deeper understanding of autoimmune disease.
This study is very strong, with well-chosen controls at every step, but has limitations characteristic of all such research. The experiments do not completely recapitulate the disease in humans, but no model will ever do that. The Xist transgene is also an incomplete construct that is expressed ubiquitously in the test subjects. But that is also why using male mice as the test case has worked: Xist RNP expression exacerbated recognizable autoimmune disease in the well-characterized pristane-induced SLE model. It has been said regarding the use of animal models to study human disease that “mice lie, monkeys exaggerate.” Sometimes. But that depends on the nature of the question asked. Here this was whether Xist RNP autoantigens contribute to autoimmune disease. The data demonstrating this in the mouse model are strong and the experimental results have been correlated with autoimmune disease markers in human disease. This is a very good start, much better than our long and winding largely futile experience with other frightening afflictions,Alzheimer’s disease being a prominent example.
Finally, the incidence of autoimmune diseases is increasing, and not only because they are now diagnosed more often than before. The robust framework produced here is likely to provide the means to evaluate putative risk factors for autoimmune disease. This is something I hope to return to soon. It has been addressed obliquely in the recently published Ultra-Processed People: The Science Behind Food That Isn’t Food by Chris Van Tulleken.
There can be no single risk factor for autoimmune disease, but in my view a good case can be made that the food-like substances of the so-called (Ultra-Processed) Western Diet are a likely culprit. We eat industrial concoctions that are digestible and organic, in the chemical sense. They only resemble food, however, as the products of modern “food science.” A simple study that could provide meaningful results would be to identify those at risk of autoimmune disease and correlate markers and disease progression with their diet.
No, correlation is not causation. That is, until a mechanism can be adduced that includes, among other things, a dose-response curve [2]. Simply by eating, the vast majority of us are getting large doses of things never before consumed by any animal. The responses are obvious, such as the obesity epidemic in countries where this diet is the default. Another of these responses may be autoimmune disease, as our bodies react to the components of our convenient and ostensibly cheap diet provided by our the “Great American Food System.”
As the title says, “science we can use” if only we will.
A brief note on biomedical research: No one should ever read a scientific paper without considering how the research was supported. From the Acknowledgments: “This work was supported by Scleroderma Research Foundation (H.Y.C.), NIAMS T32 AR007422 and NIAMS K99/R00 (D.R.D.), and NIAMS T32 AR050942 (B.T.A.). The Hopkins Lupus Cohort is supported by a grant from the National Institute of Arthritis and Musculoskeletal Diseases under award R01-AR069572. H.Y.C. is an Investigator and J.A.B. is a Hanna Gray Fellow of the Howard Hughes Medical Institute.” Nothing untoward here.
Notes
[1] Scroll down to “Research reagent” section; this technique has been used in thousands of studies of gene expression for 30+ years, including several in my laboratory.
[2] See Bradford Hill Criteria. Bradford Hill and Richard Doll showed conclusively that smoking causes lung cancer more than 70 years ago, long before the molecular mechanisms of cancer were understood.


